Figure 10. The Select Element Palette
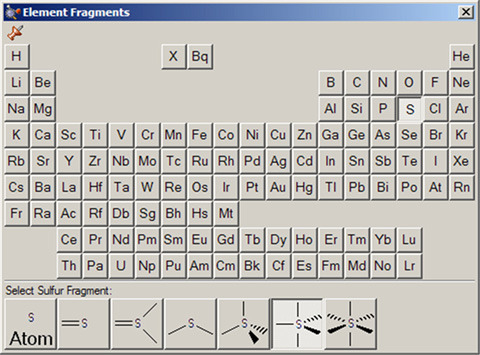
The available hybridization (connectivity) variations for the selected element appear along the bottom of the palette. In this case, the current atom type is pentavalent sulfur.
This section describes GaussView’s molecule building features. These options enable you not only to construct new structures either atom-by-atom or from fragments, but also to manipulate and examine previously-computed molecules. See the next major section of this manual, “Opening, Displaying and Saving Molecules and Views,” for information on using GaussView view features and opening and saving molecule files and images.
The GaussView molecule building tools always operate on the active view window. In general, GaussView begins and remains in its insert/replace mode, unless one of the options described below explicitly calls for it to act in a different manner. Clicking in an open area of the view window adds the current atom or fragment to the window as a distinct fragment. Clicking on an existing atom adds the current item to the existing structure at the indicated point, usually replacing it (other options are discussed later).
The desired atom or fragment is chosen using one of the Element, Functional Groups, Rings and Biological Fragments buttons and then selecting one of the choices from the resulting palette. The current item always appears in the Current Fragment area of the GaussView control window and/or the Active Fragment area of the standalone Builder palette (see Figure 3). Clicking on either the Active Fragment area on the standalone Builder palette or on the fragment name above the main window’s Current Fragment area opens the palette corresponding to the selected fragment type.
Note that the standalone Builder palette is opened with the View=>Builder menu item and can be made to always stay on top of all other open windows by checking the Builder Dialog Stays on Top checkbox in the Window Behavior Preferences (accessed with the File=>Preferences menu path).
The Element Fragments window allows you to select an element from the periodic table. The various possible coordination patterns for that element are shown at the bottom of the window (see Figure 10). The default element type is tetrahedral carbon.

Figure 10. The Select Element Palette
The available hybridization (connectivity) variations for the selected element appear along the bottom of the palette. In this case, the current atom type is pentavalent sulfur.
The Group Fragments window allows you to select from a series of AM1-optimized functional group fragments. It is illustrated in Figure 11. The default group is carbonyl (formaldehyde).
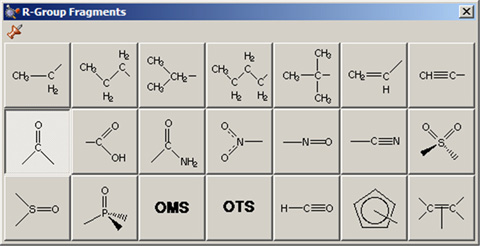
Figure 11. Select R-Group Fragment Palette
Use this palette to select the desired functional group.
The Ring Fragments window allows you to select from a series of AM1-optimized ring structures. It is illustrated in Figure 12. The default ring is benzene.
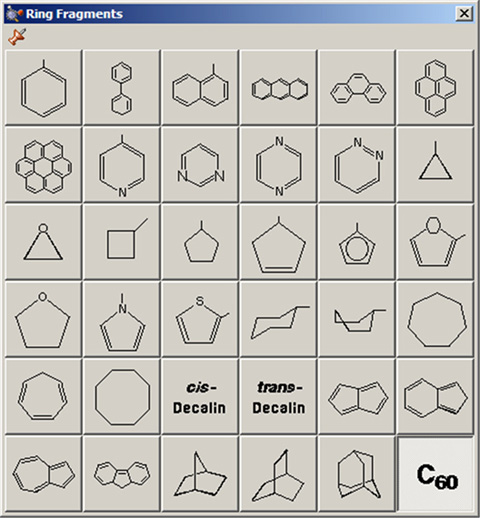
Figure 12. The Select Ring Fragment Window
Use this palette to select the desired ring system.
Note that several of the fragments use non-traditional bonding. These fragments include a five-member ring with an open bond perpendicular to the plane of the ring. This is a cyclopentadienyl ligand for use in constructing organometallic systems.
The Biological Fragments dialog presents a set of AM1-preoptimized fragments useful to biological researchers: the amino acids and various DNA bases (see Figure 13). The default biological fragment is Alanine Central Residue.
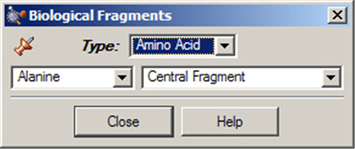
Figure 13. Selecting Biological Fragments
Use the various fields in this window to select amino acids and DNA bases.
The Type field allows you to select either Amino Acid or Nucleoside. When Amino Acid is selected, the left popup menu below the Type field can be used to select the desired amino acid, and the right popup menu in that line allows you to specify the structural form as Central Fragment, Amino-Terminal Fragment or Carboxyl-Terminal Fragment (the last two are the N-terminated and C-terminated forms, respectively).
When the Type field is set to Nucleoside, the left popup menu below the Type field can be used to select the desired DNA base, and the right popup menu in that line allows you to specify the structural form as Central Fragment, C3’-Terminal Fragment, C5’-Terminal Fragment or Free Nucleoside.
The fragment palettes in GaussView can be used in a modal (dialog box behavior) or amodal (floating palette behavior) manner. The thumbtack icon in the upper left corner of the window shows whether the palette will close when another one is selected (or a non-building context is entered). The pushed-in tack indicates that the palette is “tacked” to the desktop (i.e., “sticky”): . The upward pointing tack similarly indicates modal operation: .
The Edit=>Undo and Edit=>Redo menu paths may be used to reverse or reinstate a previous molecule modification or other action. The Undo button and the Redo button may be used for the same purposes.
The Builder toolbar/window includes the following buttons for viewing and modifying the structural parameters of molecules: Bond, Angle, Dihedral, Inquire, Add Valence, Delete Atom, and Invert About Atom. In addition, other buttons and their corresponding menu items also affect molecular structure—including Clean, Rebond, and Symmetrize—and display. We will consider these features in this section.
The Inquire button allows you to request geometric information directly from the view window when you click on the atoms of interest. Note that the selected atoms do not needed to be bonded. The structural information appears in the view window’s status bar, as in Figure 14. The number of atoms that you select affects the resulting display on the view window:
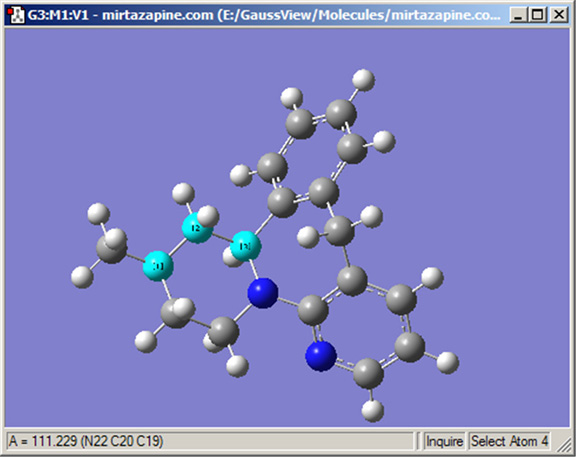
Figure 14. Inquire Mode Display
This display shows the value of the selected bond angle in the window’s lower left corner.
Three buttons, the Bond button, the Angle button, and the Dihedral button, allow rapid and intuitive modification of geometric components. Once selected, the atoms of the bond in question must be selected for the respective SmartSlide dialog box to open. Once you have modified a parameter using the SmartSlide, the OK button must be pressed before any of the changes made on any SmartSlide are actually applied to the molecule. Clicking the close box will discard any changes (as will selecting the Cancel button).
The Bond SmartSlide is illustrated in Figure 15. The initial bond length value is set to the distance between the selected pair of atoms, and the type of bond existing between them is indicated. The SmartSlide provides access to all the possible bond types.
The interatomic distance is dynamically adjusted by moving the slider along the scale. Values may also be directly entered in the text box.
You can change the bond type by clicking one of the bond type radio buttons without affecting the valence on the atoms from which the bonds are removed. Note that this is a purely visual exercise, as quantum mechanical methods like those in Gaussian do not take account of connectivity, but rather determine bonding from the wavefunction.
Note that the Bond SmartSlide is also used to add bonds between unbonded atoms. Simply click on the two atoms to be bonded as usual. When the SmartSlide opens, click on the type of bond desired, and the bond will be created. Similarly, to remove a bond, select the two atoms you want to unbond, and then select the None radio button in the dialog.
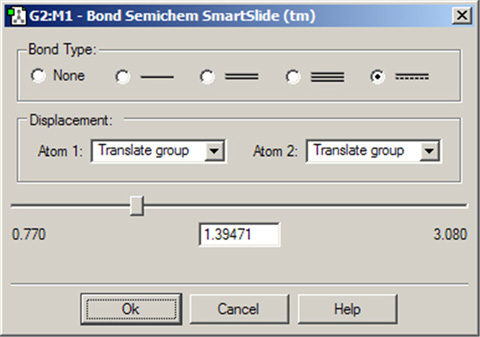
Figure 15. The Bond SmartSlide
This dialog can be used to add, remove and change bond lengths.
The Displacement fields specify how attached groups are handled as the bond distance changes:
Figure 16 illustrates the effects of different combinations of these choices.
 |
 |
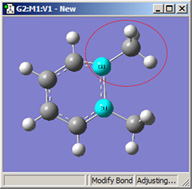 |
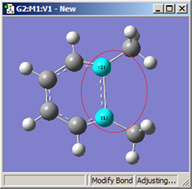 |
| Original | 1=Fixed, 2=Translate Atom | 1=Fixed, 2=Translate Group | 1,2=Translate Group |
Figure 16. Bond Length Modification Options
All three of these structures result from lengthening the bond distance between the two selected C atoms (to the same value in all three cases). In the left picture, atom 1 was held fixed, and atom 2 was set to Translate Atom. Compare this to the middle picture which also held atom 1 fixed and set atom 2 to Translate Group. The upper methyl group stays fixed in the first case, while the 1-2-R bond angle remains unchanged in the center case. The right picture sets both atoms to Translate Group, probably the most common choice for a case like this one.
Figure 17 illustrates the dialog associated with the Angle SmartSlide. The dialog for the Dihedral SmartSlide is similar, but it includes Displacement fields only for Atom 1 and Atom 4.
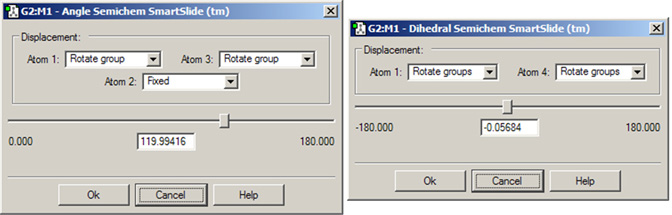
Figure 17. Angle and Dihedral SmartSlides
These dialog modify bond angles and dihedral angles (depending on how many atoms are selected).
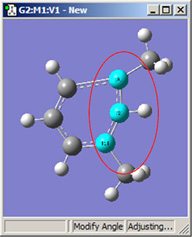
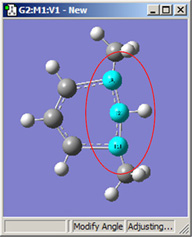
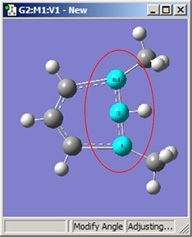
Figure 18 illustrates some of the combinations of various Displacement settings for bond angles (using the same original molecule as Figure 16). The possible choices are:
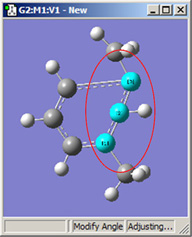 |
 |
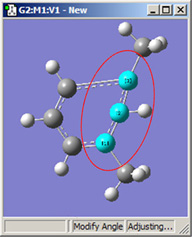 |
| 1,2=Fixed, 3=Rotate Atom | 1,2=Fixed, 3=Rotate Group | 1,2=Fixed, 3=Translate Group |
 |
 |
 |
| 1,3=Rotate Atom, 2=Fixed | 1,3=Rotate Group, 2=Fixed | 1,3=Translate Group, 2=Fixed |
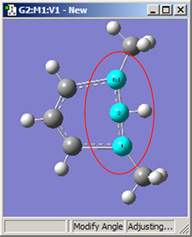
Figure 18. Bond Angle Modification Options
All six of these structures result from increasing the 1-2-3 bond angle. In the top row, atoms 1 and 2 are both held fixed. Compare the results of increasing the bond angle to the same value in the three cases (keeping in mind the “normal” position for the ring atoms and the methyl groups). The movement of the methyl group attached to atom 3 ranges from substantial in the first frame to a little in frame 2 to almost none in frame 3. In the second row, atom 2 is held fixed while other choices are used for atoms 1 and 3. Comparison of these illustrations as well as experimentation will make the differences between the choices clear and familiar.
The Add Valence button attaches additional valences (shown as hydrogen atoms) to the center clicked with the mouse. The new valences will be placed as far as possible from the other atoms attached to the center.
Similarly, the Delete Atom button removes atoms and open valences. When used, the function will eliminate the selected atom and all single valences (hydrogens) attached to it. It may also be used to remove a dangling bond by clicking on the bond stick (see Figure 19).
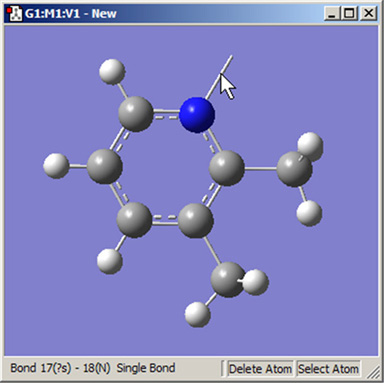
Figure 19. Deleting a Dangling Bond (Open Valence)
The Delete Atom button may be used to remove atoms and dangling bonds. An example of the latter is shown at the top of the molecule. Clicking on it will remove the open valence.
The Invert About Atom button allows you to reverse the symmetry of a molecule with respect to a selected atom. Figure 20 illustrates the use of this feature.
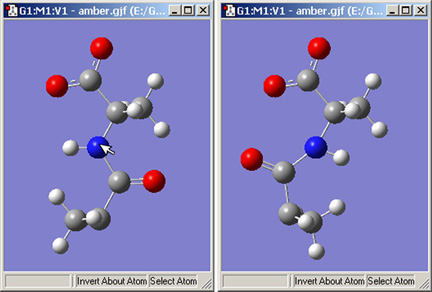
Figure 20. Inverting a Structure About an Atom
Clicking on the nitrogen atom with the Invert About Atom tool will result in the structure on the right.
The Clean button and Edit=>Clean menu item both adjust the geometry of the molecule, based on a defined set of rules, to more closely match chemical intuition. The results are only approximations and are not intended to be perfect. Geometries may require adjustment for non-classical cases such as transition states.
The GaussView clean function may be customized via the Clean Controls preferences panel (illustrated in Figure 21).
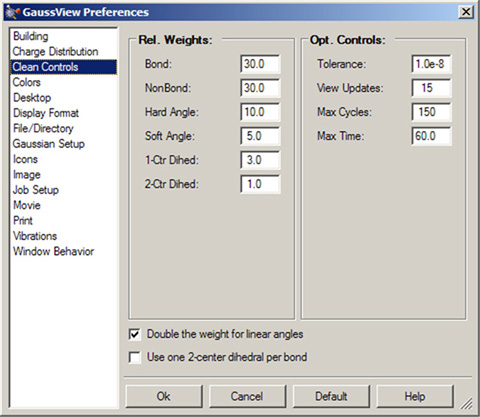
Figure 21. Customizing the Clean Function
You can use this preference panel to modify and customize how the GaussView clean function operates.
The default clean settings attempt to achieve a balance that produces expected “normal” results. Altering the settings in Clean Controls preferences can cause unexpected and undesirable results, so please read the following sections carefully before making any changes.
The following controls are available in the dialog. The fields in the Rel. Weights column are:
The checkboxes at the bottom of the window have the following effects:
The fields in the Opt. Controls column are:
It is important to remember that the various components of the clean force field are relative. Changing one weight will affect the behavior of the other weights. For example, excessive non-bond weight will produce longer bonds. Similarly, an excessive hard angle weight could affect the 2-center dihedrals.
You can disable any set of terms by assigning a weight of 0.0. For example, disabling the 1-center dihedral term can result in faster cleaning, but the resulting tertiary structure may not be as nice. Do not disable the bond weight or hard angle terms. The former should be kept high relative to the other terms.
Clean Performance Tips
If the clean function is too slow on your system, try these settings:
Poor performance of the clean function can also be a symptom of a memory shortage on the system.
The Rebond button and Edit=>Rebond menu path both initiate a rebonding process in which GaussView reidentifies bonded atoms, based on a distance algorithm. Note that Gaussian does not use the bond information of the screen for calculations. This information is presented only to make it convenient for you to visualize the chemistry of the molecule.
The Point Group Symmetry dialog is used to specify the desired symmetry for a molecular structure (see Figure 22). It is reached via the Edit=>Point Group menu path. The controls have the following meanings:
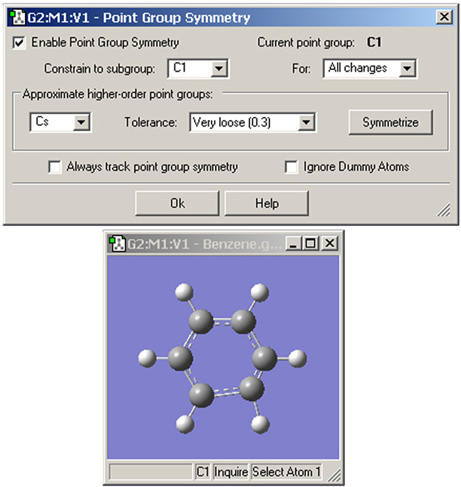
Figure 22. Imposing Symmetry on a Molecular Structure
In this example, GaussView has identified the current point group of this distorted benzene molecule as C1. It also recognizes that the C2v point group might also be appropriate. Clicking the Symmetrize button will apply the specified point group to the structure. The Tolerance field can be used to tighten or relax the symmetry identification process. In this case, loosening the tolerance allows GaussView to identify the highest applicable point group as D6h.
The Symmetrize button (on the toolbar) and the Edit=>Symmetrize menu item both immediately impose the maximum identifiable point group on the current structure, using the most recent Tolerance setting from the Point Group Symmetry dialog for this molecule (or the default of Normal). They also have the side effect of enabling point group symmetry for the molecule (again, using the previous settings, if any).
The view window status bar will indicate any identified and constrained symmetry for the molecule, as illustrated in Figure 23.
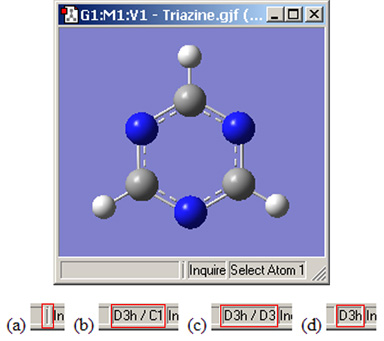
Figure 23. Symmetry-Related Status Bar Displays
Four views of the symmetry information section of the status bar for the triazine molecule: (a) symmetry has not been turned on; (b) molecule symmetry (D3h) but no symmetry constraint (C1); (c) molecule symmetry (D3h) and structure constrained to D3 symmetry; and (d) molecule symmetry and constraint symmetry are both D3h.